Chemical structure:
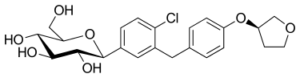
IUPAC name:
(2S,3R,4R,5S,6R)-2-[4-Chloro-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
CAS number:
864070-44-0
Therapy area:
Anti-diabetic
References:
IH
Status:
Under development
Mechanism of action
The sodium glucose co-transporter-2 (SGLT-2) is almost exclusively present in the proximal tubules of nephronic components in the kidneys, and empagliflozin is an inhibitor of SGLT-2. Around 90% of the blood’s reabsorption of glucose is mediated by SGLT-2. Blocking SGLT-2 decreases blood glucose by inhibiting glucose reabsorption in the kidney and so excreting glucose (i.e., blood sugar) via the urine.
DISCLAIMER: PRODUCTS COVERED BY VALID & UNEXPIRED PATENTS ARE NOT OFFERED (OR) SUPPLIED FOR COMMERCIAL SCALE. THE PATENT POSITION SHOULD BE VERIFIED & LIABILITY LIES WITH THE CUSTOMER ONLY. PRODUCTS COVERED BY PATENTS ARE AVAILABLE ONLY FOR R&D USE.




