Chemical structure:
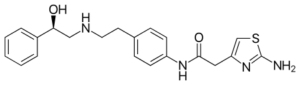
IUPAC name:
2-(2-Amino-1,3-thiazol-4-yl)-N-[4-(2-{[(2R)-2-hydroxy-2-phenylethyl]amino}ethyl)phenyl]acetamide
CAS number:
223673-61-8
Therapy area:
Overactive Bladder
References:
IH
Status:
Under development
Mechanism of action
After oral administration, mirabegron is rapidly absorbed, with a time to maximum plasma concentration (Tmax) of around 3 hours and a terminal plasma half-life of 50 hours. The majority of the substance—roughly 70%—is bonded to albumin and then to 1-acid glycoprotein, which are both plasma proteins. Oral bioavailability ranges from 24% to 53%, depending on dosage and gender. With a dosage of 50 mg, the absolute bioavailability rises from 29% at a dose of 25 mg to 35%.
DISCLAIMER: PRODUCTS COVERED BY VALID & UNEXPIRED PATENTS ARE NOT OFFERED (OR) SUPPLIED FOR COMMERCIAL SCALE. THE PATENT POSITION SHOULD BE VERIFIED & LIABILITY LIES WITH THE CUSTOMER ONLY. PRODUCTS COVERED BY PATENTS ARE AVAILABLE ONLY FOR R&D USE.


